Anisotropic Nanostructures
As materials are reduced in size from the bulk to the nanoscale, they begin to exhibit new and unusual properties and functionalities. Noble metal nanostructures are of particular interest because such characteristics can be controlled by varying composition, size, and shape. Anisotropic nanoparticles – non-spherical structures (e.g., prisms, rods, cubes) – have shape-dependent chemical and physical properties and can be utilized in important applications spanning catalysis to sensing to optics.
In the Mirkin group, one of our primary goals is to develop synthetic strategies for creating diverse libraries of structurally uniform, anisotropic noble metal nanoparticles in high yield with fine control over shape and size. We focus mainly on two types of methodologies: seed-mediated and photo-mediated synthesis. Post-synthetically, anisotropic nanoparticles can be coated with a dense shell of oligonucleotides to form “programmable atom equivalents” (PAEs), where directional DNA “bonds” can be used to program the arrangement of nanoparticle “atoms” into one-, two-, and three-dimensional colloidal crystals with emergent properties.
Seed-Mediated Synthesis
Seed-mediated syntheses utilize nanoparticle reactants, or seeds, as templates for the heterogeneous nucleation of anisotropic nanoparticle products. In this way, the processes of seed nucleation and subsequent nanoparticle growth can be separated to allow for better control of each step, leading to the synthesis of more uniform particles. Research in our group has focused on tuning the reaction conditions required to control shape, with a specific emphasis on the use of halides and silver (via underpotential deposition) as shape-directing agents. This work led to the delineation of design rules for how to control shape and the preparation of nanoparticles with novel morphologies (Fig. 1), including concave cubes, which are composed of 24 high-index facets, {110}-faceted bipyramids, and octahedra with hollow features.

Figure 1. High-quality seeds can be used interchangeably to generate eight different shapes. Each panel represents a different shape synthesized and is arranged counterclockwise from top left as three-dimensional graphic rendering of the shape; TEM image (scale bars are 100 nm); high-magnification SEM image of crystallized nanoparticles (scale bars are 500 nm) with FFT pattern inset. Moving clockwise from the top left, the shapes described are cubes, concave rhombic dodecahedra, octahedra, tetrahexahedra, truncated ditetragonal prisms, cuboctahedra, concave cubes, and rhombic dodecahedra. (Taken from J. Am. Chem. Soc. 2014, 136, 7603–7606.)
Subsequent research focused on unraveling the mechanisms behind seed-mediated syntheses to enable unprecedented control over the structural uniformity and yield of anisotropic nanoparticles. Others in the field were focused on how to transform an ill-defined initial state (seeds less than 5 nm in size) into a well-defined end product (uniform high-quality particles) through manipulation of reaction conditions; we have instead taken a different approach – developing chemistry to control the uniformity of the seeds. This work was guided by the hypothesis that the quality of the seeds would impact the quality of the products. We reported how iterative reductive growth and subsequent oxidative dissolution could be used for the stepwise refinement of gold nanoparticle seeds used for anisotropic particle synthesis.
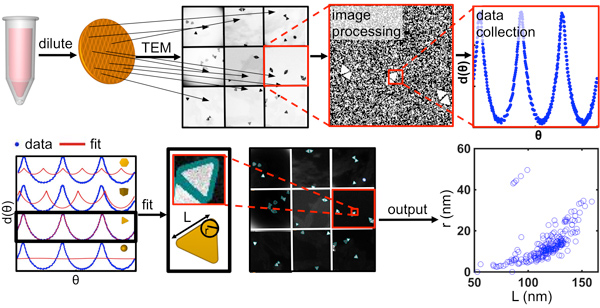
This novel capability allowed us to systematically study how the size dispersity, shape variation, and crystalline structure of the seed influence anisotropic nanoparticle products. To this end, we synthesized eight classes of single-crystalline nanostructures from the same batch of seeds, each consisting of a different shape, where the shape and size uniformity exceeds that of all previously reported syntheses. Subsequently, we employed the principles elucidated in this work to two-dimensional particles, where a non-uniform mixture of triangular, truncated triangular, and hexagonal plates could be oxidized in a self-limiting, tip-selective reaction that converts each of these products into similarly sized circular disks, resulting in considerable particle homogenization and narrower plasmon resonances. As part of this work, we developed software to algorithmically analyze electron microscopy images, allowing for the rapid quantification of the size and shape distribution for different nanoparticle populations (Fig. 2).
Figure 2. Overview of the process for quantitative analysis of nanoparticle structure from electron microscopy (EM) images, including EM methods and computational processing. In particular, the most appropriate EM data collection requires dilute sample preparation and acquisition of images at diverse grid locations. Computationally, the program processes raw EM images and extracts angular distance data, d(θ), from individual particles. It then performs a shape fit and repeats the process iteratively for multiple images to produce population statistics. (Taken from ACS Nano 2015, 9, 12488-12495.)
However, with most traditional seed-mediated approaches, the spherical or anisotropic seeds act as nuclei due to the absence of significant energy barriers (E) for subsequent growth. Consequently, secondary nucleation does not occur, and instead, growth occurs at all positions on the seeds. We recently developed a versatile strategy that allows one to control growth on seeds in a site-specific manner (Fig. 3). If one analyzes the surface chemistry of a non-spherical seed, such as a triangular prism, one discovers that there are distinct energy barriers to additional growth due to differences in ligand density (caused by differences in curvature on different regions of the surface; i.e., flat facets versus edges versus corners) and the exposed facet type. Therefore, through the use of appropriate ligands, significant E differences on anisotropic seeds can be established, allowing one to access corner-, edge-, or facet-selective growth while tuning the supersaturation (∆μ) of the growth solution (the driving force) to only surmount certain E. In addition, we found that further fine-tuning of ∆μ not only dictates the site of deposition (e.g., the edge or corner of an octahedron) but also determines the exposed facets (e.g., {100} or {111}) of the overgrown regions, thus allowing control over the overall shapes of the resulting nanocrystals. Based on this principle, we have synthesized over 10 here-to-fore never reported nanocrystals and, in principle, many others are possible.
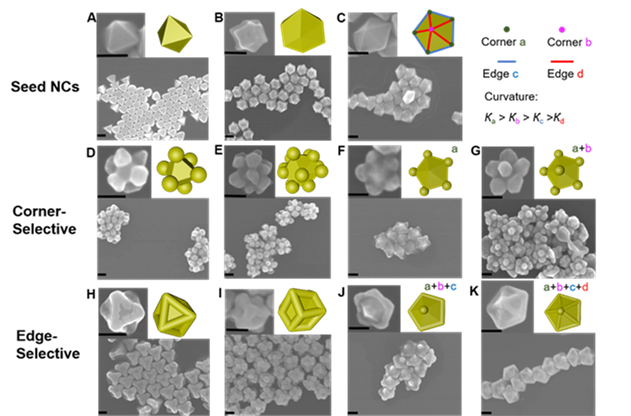
Figure 3. Curvature-selective nucleation on different-shaped seed NCs. (A-C) SEM images and models (inset upper right) of octahedral, concave rhombic dodecahedral, and decahedral seed NCs, respectively. SEM images and models (inset upper right) of (D–G) corner- and (H-K) edge-selective nucleation on the seed NC shown in the top row. Decahedral NCs (C) contain two types of corners (a & b) and two types of edges (c & d) due to curvature differences such that Кa > Кb > Кc > Кd, which result in four levels of continuous but independent control of selective nucleation as seen in panels F,G,J,K. Scale bars: 100 nm. (Taken from Science Advances 2021.)
Photo-Mediated Synthesis and the Concept of Plasmonic Seeds
Anisotropic nanoparticle synthesis research in the Mirkin group began with our discovery and development of a photo-mediated (also referred to as a plasmon-mediated) synthesis for silver triangular nanoprisms, one of the first high-yield anisotropic nanoparticle syntheses ever developed (Fig. 4). We have shown that triangular nanoprism morphology can be controlled photochemically by adjusting the wavelength of irradiation or chemically by varying the pH of the reaction solution. Photo-mediated synthesis also can be used to generate Au-core/Ag-shell structures by seeding the reaction with Au particles and irradiating the reaction solution at a wavelength equal to the plasmon resonance of the Au core. In this way, these particles serve as “plasmonic seeds” where light can be used to control particle growth and resulting shape. By changing the Ag precursor in the photo-mediated synthesis from Ag nanospheres to AgNO3, we have synthesized a variety of additional particle morphologies, such as right-triangular bipyramids and penta-twinned rods (Fig. 4). Through this work, we have begun to work out the mechanisms that underlie these unusual transformations.
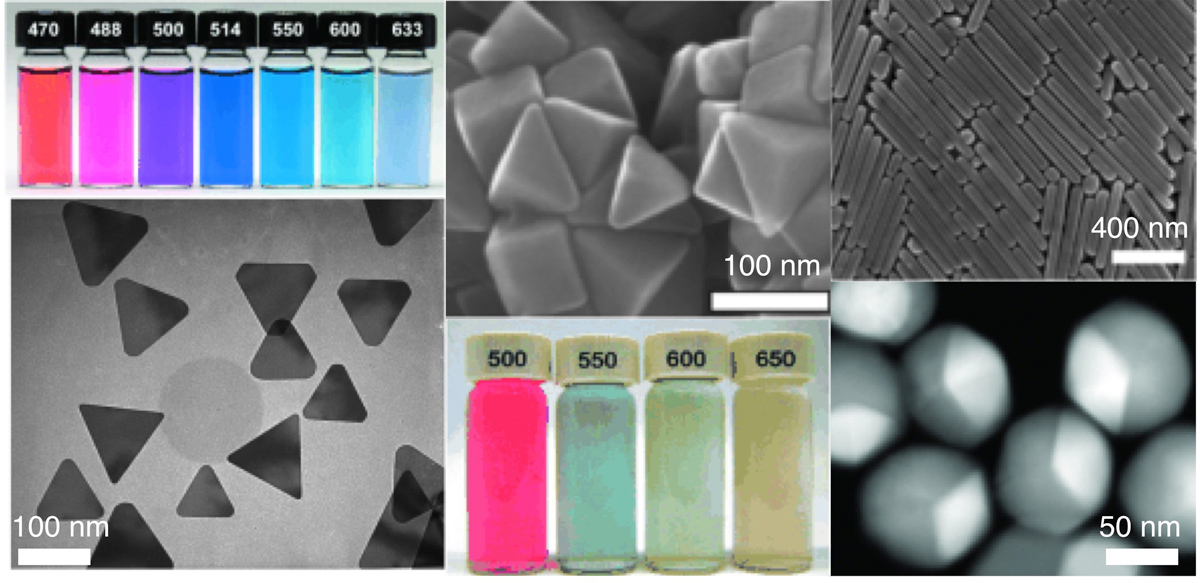
Figure 4. Silver nanostructures prepared by plasmon-mediated syntheses. Left column: Solutions of triangular nanoprisms prepared at different excitation wavelengths and a representative transmission electron micrograph. (Adapted from Angew. Chem. Int. Ed. 2007, 46, 2036-2038.) Center column: Scanning electron micrograph of right triangular bipyramids and solutions of bipyramids prepared by different excitation wavelengths. Right column: Penta-twinned nanorods (top) and gold-core/silver-shell bimetallic icosahedra (bottom).
Anisotropic Nanoparticle Assembly
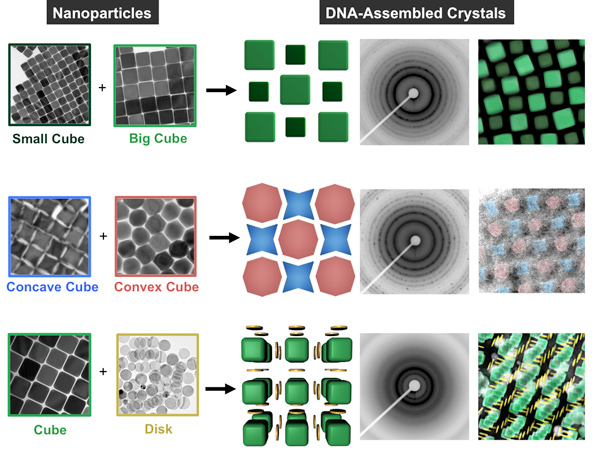
With this library of anisotropic nanoparticle building blocks in hand, we begun to investigate their use in a number of applications, ranging from plasmonics to assembly. Assembly represents a particularly interesting avenue of research because shape can be used to introduce directional interactions between particles. For example, we investigated the effect of shape in the context of DNA-mediated nanoparticle crystallization (Fig. 5). In this work, DNA is used as a surface ligand to mediate interparticle interactions through sequence-specific hybridization events. The DNA forms a densely packed, conformal shell around the nanoparticle, such that the underlying shape plays an important role in controlling the strength, number, and directionality of the DNA “bonds” for a given particle.
Our initial work showed that nanoparticles of a given shape (e.g., triangular prisms, rods, octahedra, rhombic dodecahedra) will crystallize with a lattice symmetry that maximizes the number of face-to-face interactions between particles (thus maximizing the number of DNA hybridization events). We have subsequently investigated how the DNA bond strength between particles changes as a function of shape and how differences in bonding strength can be exploited to separate otherwise difficult-to-purify mixtures. In addition, we demonstrated the selective co-crystallization of mixtures of two different anisotropic nanoparticles and investigated the effects of nanoparticle size and shape complementarity on the resultant crystal symmetry, microstrain, and effective ‘DNA bond’ length and strength.
Figure 5. Nanoparticles with different shapes and sizes (left two columns) can be selectively cocrystallized with complementary DNA sequences, seen in drawings (center column) and false-colored TEM images (right). Small-angle x-ray scattering produced a characteristic diffraction pattern for each type of crystal (second from right). (Adapted from Nature Materials 2015, 14, 833-839.)
Recently, we also investigated how magnetic interactions between nanoparticles can be coupled with DNA interactions to drive assembly. Cubic Fe3O4 nanoparticles exhibit assembly behavior that is a consequence of a competition between magnetic dipole-dipole and ligand interactions. We functionalized the surfaces of cubes with DNA to program face-to-face interactions, and we investigated their assembly both with and without an applied magnetic field. Upon application of a field, a tilted orientation of cubes arises, enabled by the flexible DNA ligand shell. This led to an unexpected crystallographic alignment of the entire superlattice, as opposed to just the individual particles, along the field direction as revealed by small and wide-angle X-ray scattering (Fig. 6). This observation is dependent upon DNA length and sequence and cube dimensions. Taken together, these studies show how combining physical and chemical control can expand the possibilities of crystal engineering with DNA.
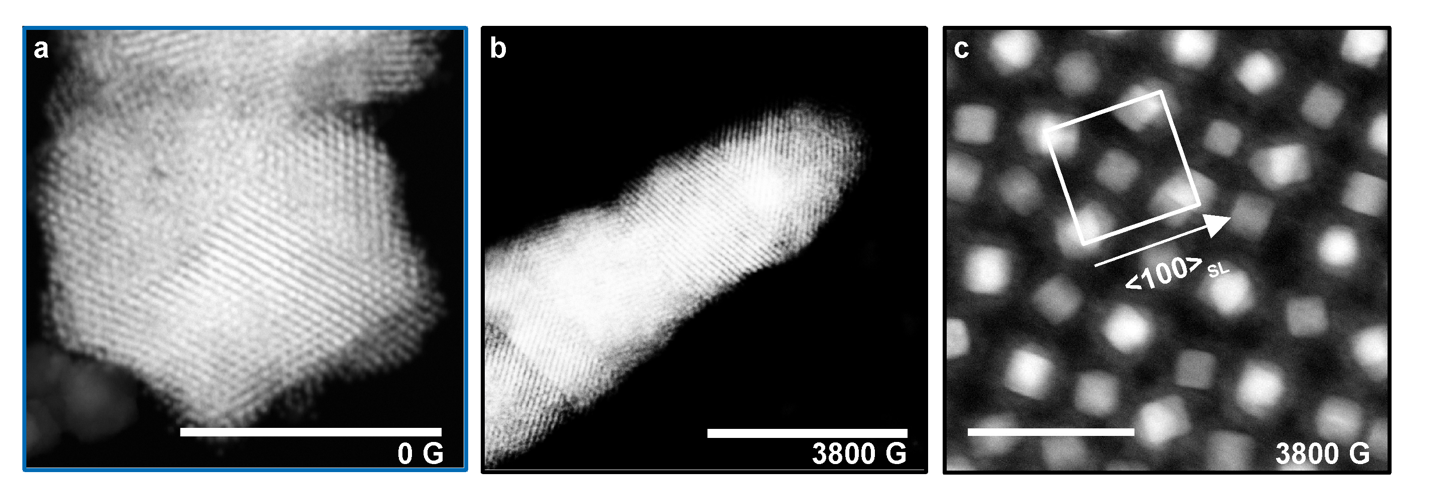
Figure 6. Scanning transmission electron microscopy (STEM) Z-contrast images of silica-embedded assemblies of magnetite cubes grown at a field strength of a) 0 G and b) 3800 G. Scale bars 1 μm. Without a field, faceted crystals form due to the isotropic arrangement of DNA and with a field elongated rods form due to the magnetic alignment to an external field. c) STEM Z-contrast images of cross-sectioned assemblies grown at 3800 G show the tilted arrangement of cubes with respect to the particle lattice. Scale bar 100 nm. (Adapted from Angew. Chem. Int. Ed. 2020, 59, 1-6.)
Using DNA-mediated nanoparticle adsorption, we have also constructed optical metasurfaces of ordered arrays of anisotropic nanoparticles (Fig. 7), allowing for the systematic control of photonic and plasmonic coupling modes in a single device. This work has implications for the development of advanced nanophotonic structures in sensing and quantum plasmonics and as tunable absorbers.
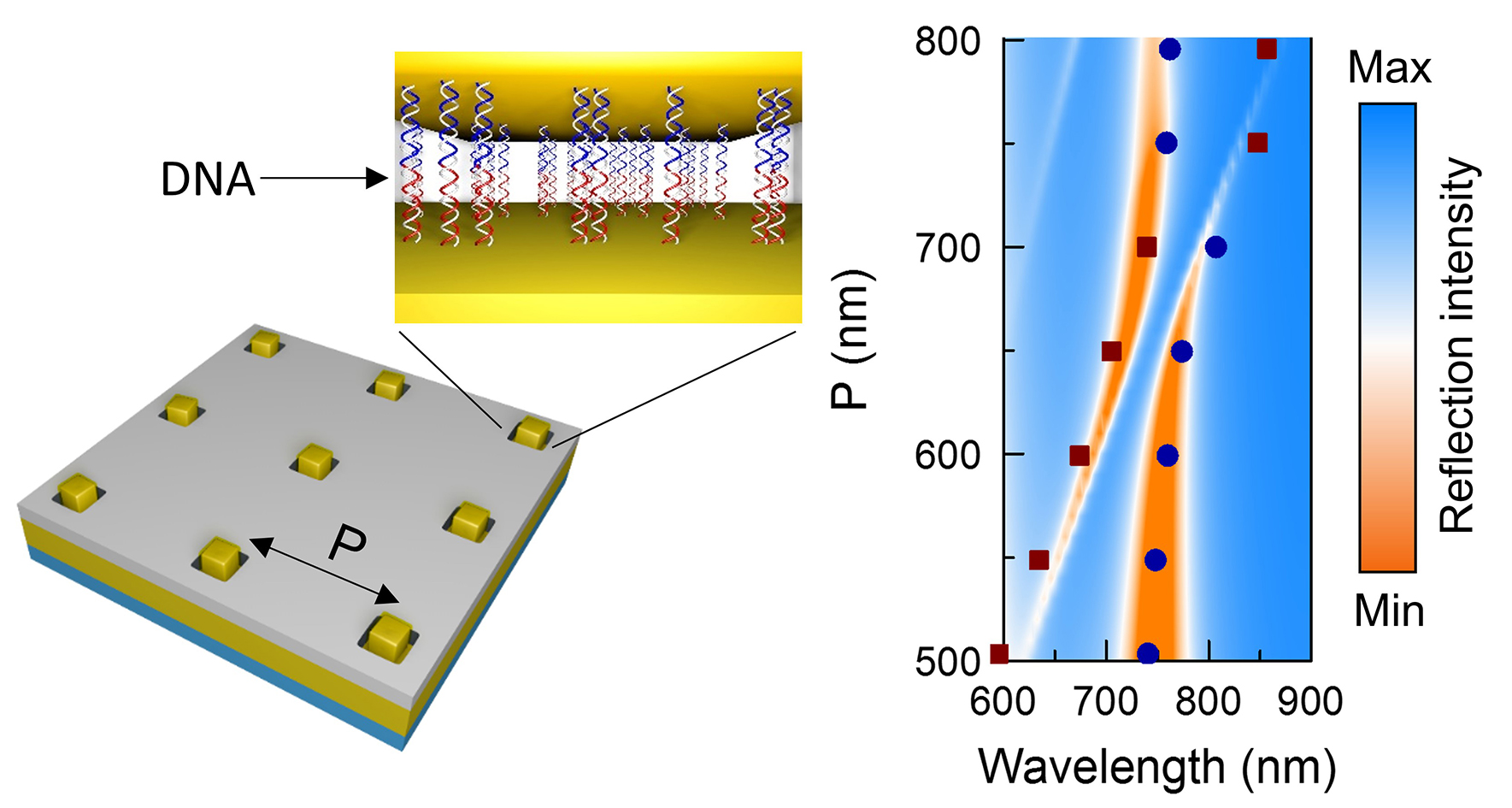
Figure 7. Optical metasurfaces with control of both photonic and plasmonic coupling can be constructed using an approach that combines top-down and bottom-up processes, wherein gold nanocubes are assembled into ordered arrays via DNA hybridization events onto a gold film decorated with DNA-binding regions defined using electron beam lithography.
Anisotropic Nanostructures Subgroup Members

Back Row (L-R): , Yi Xie, Zhiwei Li, Chaojian Chen, Cuizheng Zhang, Jarod Beights, Yiming Yang, Zifeng Lin, Jaewon Lee
Front Row (L-R): Alexa Wong, Xiaowei Liu, Zihao Ye, Jeongmin Cho, Ana Leal, Rachel Chan (subgroup leader), Ye Zhang (subgroup leader), Adam Claman, Olivier Chevalier, Arindam Raj